Cancer is the attributed cause of death in one in four cases in the United States, and metastasis—a complex multistep process leading to the spread of tumors—is responsible for more than 90% of these deaths. However, predicting the location of secondary tumor sites remains an elusive goal. Achieving this requires a precise understanding of cancer cell transport through the vascular system, including their likelihood of penetrating the vessel wall. Such predictive capability would enable three major benefits: (1) supporting a more targeted search for metastases during initial cancer staging; (2) predicting the primary tumor site for patients with metastatic disease of unknown origin; and (3) informing the design of next-generation nanotechnologies to target circulating tumor cells (CTCs) in the bloodstream.
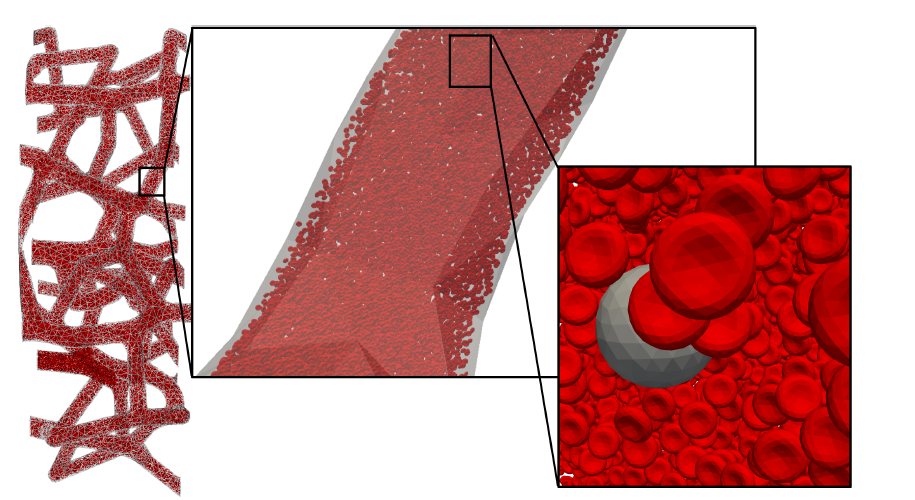
Our interest in cancer cell migration builds on our long-standing focus on fluid–structure interactions (FSI) and their role in disease localization. We employ the lattice Boltzmann method (LBM) as the computational fluid dynamics core of HARVEY, our massively parallel vascular flow solver, and couple it with immersed boundary methods (IBM) to capture the deformable mechanics of CTCs, red blood cells, white blood cells, and platelets. To address the challenge of simulating these complex interactions across scales—from micron-level cell deformation to meter-scale vascular transport—we integrate our adaptive physics refinement (APR) framework. APR dynamically increases model fidelity only where and when it is needed, enabling us to capture fine-scale adhesion and collision events while maintaining computational efficiency.
Using this multiscale FSI–APR approach, we can study how flow patterns, residence times, and cellular interactions influence CTC adhesion and extravasation, both of which are critical precursors to metastasis. This framework provides a pathway toward identifying vascular regions most likely to host secondary tumor sites, on a per-patient basis. Below, we show an example from a recent publication examining the transport of a compound capsule through a constricted microchannel, illustrating how material properties and local hemodynamics can alter migration and adhesion potential.
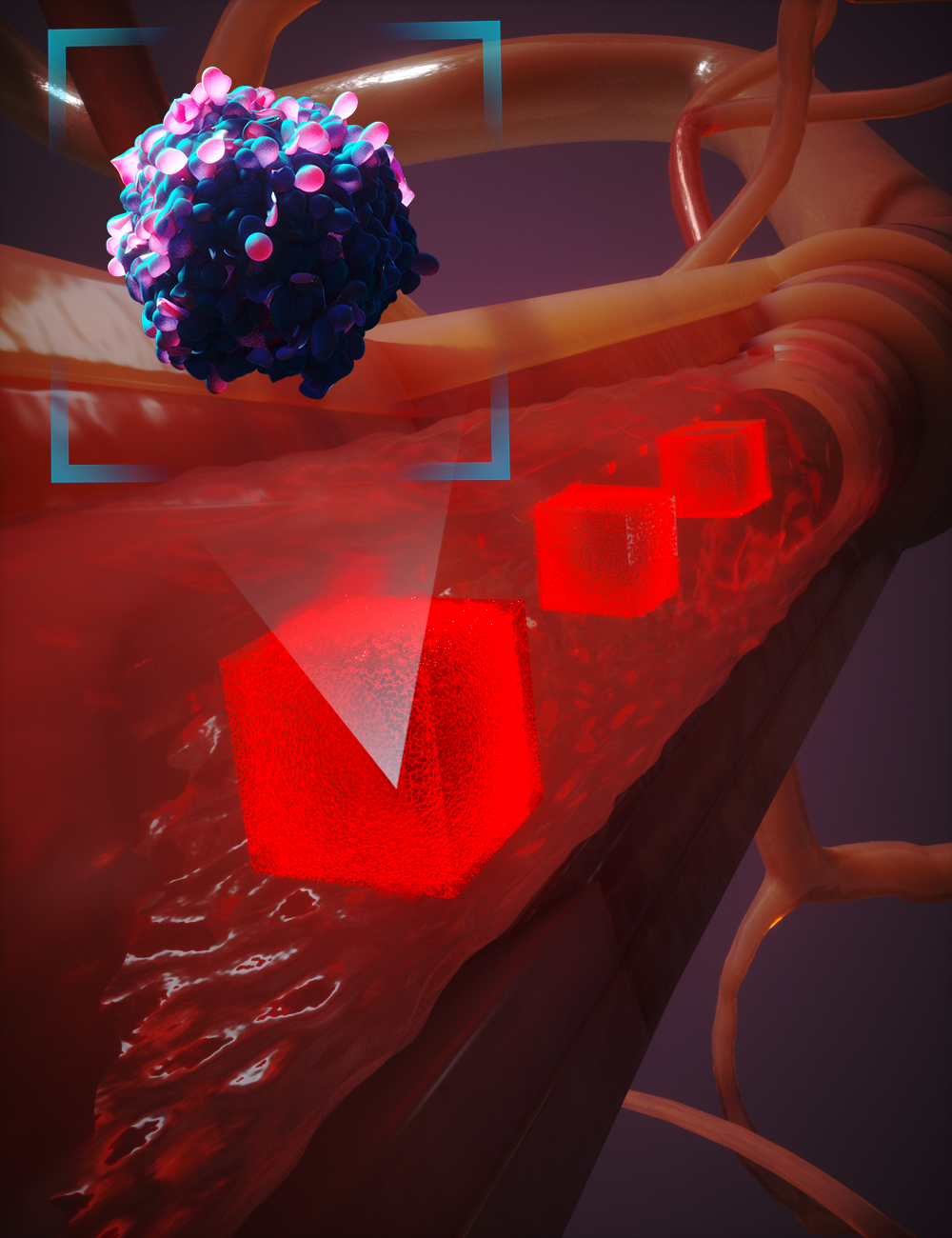
The figure above illustrates APR’s “moving window” concept in action. Rather than applying the highest-resolution physics to the entire vascular domain, APR continuously tracks areas of interest—such as a circulating tumor cell approaching a vessel constriction—and refines the model only within that localized window. As the event progresses, the high-fidelity zone moves with it, ensuring critical interactions are resolved in detail while the surrounding regions are simulated at a coarser scale. This targeted refinement dramatically reduces computational cost without sacrificing accuracy in the moments and locations that matter most.
